Rare Diseases in India: ‘Orphan’ No More?
Introduction: The Struggle for Definitions
Medical experts have yet to reach a consensus on a universal definition of what constitutes ‘rare diseases’. The World Health Organization’s (WHO) working definition states that these diseases are debilitating, lifelong disorders whose prevalence is less than one per 1,000 persons. They include autoimmune disorders, congenital malformations, inherited cancers, and certain endemic infectious diseases that have very low prevalence. Around 80 percent of rare diseases are genetic in origin and therefore disproportionately impact children.
Globally, the prevalence threshold of these diseases ranges from five to 76 per 100,000 people across countries Even as these diseases are, individually, rare, they collectively affect a significant 10 percent of the global population.
To obtain regulatory and financial incentives to develop rare disease therapies, drug development agencies have also come up with their own definitions and eligibility criteria. These vary, as the overall bucket of conditions determining such diseases is spread unevenly across geographies, and is also influenced by factors such as social determinants and genomic structures. A disease that is rare in one particular demography or region may not be so in others. Such inter-regional disparities are found even within countries.
In India, for instance, some studies have reported a higher prevalence of β-thalassemia in pockets of Gujarat, Punjab, West Bengal, Odisha, and Andhra Pradesh than in the rest of the country. Similarly, Duchenne Muscular Dystrophy is a rare disease, but it has a higher-than-benchmark prevalence in Tamil Nadu.
Countries define rare diseases in the context of their own populations, healthcare systems, and resources. Most developed countries have adopted a uniform definition to facilitate research and development, and policymaking. India, like many developing countries, is yet to formalize its definition.
The Burden and Management of Rare Diseases
About 80 percent of rare diseases are believed to be genetic in origin, while 70 percent start in childhood. About 9 percent of Southeast Asia’s population suffer from them. The cultural practice of consanguineous marriages is believed to be one of the reasons for the high prevalence of rare diseases in this region. In India too, endogamy in the north and consanguinity in the south are said to be responsible for many of the rare diseases.
As India does not have a standard definition of rare diseases, it is difficult to arrive at their exact prevalence. The approximate number, though, is that 72 to 96 million people in India suffer from rare diseases. Epidemiological data for rare diseases is collated by the National Registry for Rare Diseases, maintained by the Indian Council of Medical Research (ICMR). Until 31 October 2021, 4,001 different rare diseases had been recorded; the most common among them are Haemophilia, Thalassemia, sickle cell anaemia, primary immune-deficiency in children, autoimmune diseases, Lysosomal storage disorders (such as Pompe disease), Hirschsprung disease, Gaucher’s disease, Cystic Fibrosis, Hemangioma, and some forms of muscular dystrophy.
Only 5 percent of rare diseases worldwide are treatable. A vast majority do not have specific targeted treatment, and patients often receive functional therapies to alleviate symptoms. Some also require lifelong administration of antidotes and supportive medication, which results in high direct and indirect costs compared to other diseases. Poor treatment facilities, delayed diagnosis, and dependency on imported drugs are some of the significant factors that lead to high treatment costs. Early and accurate diagnosis is vital for proper management of rare diseases. This remains a key challenge worldwide, leading to delayed therapy and poor prognosis. On average, after the onset of symptoms, it takes at least seven years on average for rare diseases to get diagnosed in India, and in many cases, such diseases remain misdiagnosed, if at all.
The National Policy for Rare Diseases provides that, for those diseases that it lists as “rare”, those below the poverty line are entitled to a one-time government assistance of INR 2,000,000 for curative treatment. This means that those who may be above the poverty line by definition, but are poor, do not receive any subsidy. The high costs of procedures like organ transplant or Hematopoietic Stem Cell Transplant (HSCT)—used to treat certain rare diseases—could often push these families into poverty. Further, diagnostic capabilities at government health centres, particularly the primary and secondary ones, are almost negligible, forcing families to spend a fortune at private facilities for years just to arrive at a diagnosis.
Given the high costs, crowdfunding could be one of the few viable options of paying for rare disease treatments. Crowdfunding takes time, however, and the delay itself can cause irreversible damage to patients with progressive conditions. The national policy calls for a centralised crowdfunding platform for a relatively small group of rare diseases, whose treatment is expensive and lifelong (those defined by the policy as ‘Group 3 diseases’). But the platform has a limited reach, and engagement with stakeholders is deficient. In more than a year since its launch, the platform has raised a meagre INR 118,016 for 253 patients.
With rare diseases, patient advocacy groups (PAGs) also play a significant role in fast-tracking regulatory approvals, influencing policies, fostering collaborative partnerships with industries, alleviating the burden on caregivers, and leading crowdfunding initiatives. There are many instances where PAGs have initiated or supported research programmes, which have led to the development of new treatment for diseases like cystic fibrosis, lymphangiomyomatosis, and Pompe’s disease. One such example is the Metabolic Errors and Rare Disease Organisation of India (MERD) which advocates a special diet for children with inborn errors of metabolism (IEM). As a result of their advocacy efforts, the Food Safety and Standards Authority of India (FSSAI) launched a ‘Diet4Life’ initiative in 2017, focused on early screening of babies for IEM, formulating diets for them, and making these foods widely available in the country at subsidised rates.
Figure 1: Roles and Responsibilities of Patients and Caregivers
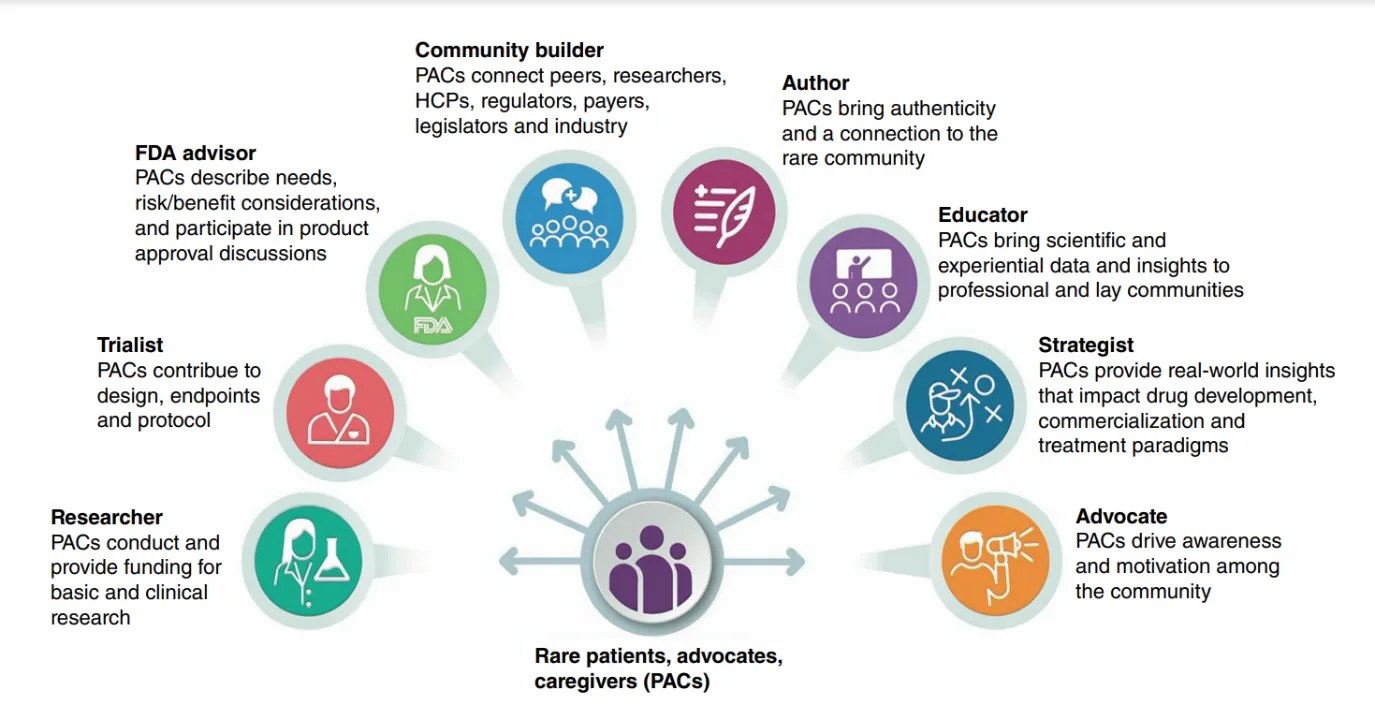
Figure 1 presents a range of roles that patients and caregivers can play when advocating for more attention to the disease. Prioritizing patient-centric care, and ensuring that drug development is a co-development effort helps design empathetic products and systems that help relieve families of stress. Additionally, caregivers are key in the participation and adherence of patients to relief therapies and scientific trials for drug development. Qualitative inputs from them allow the identification of what will work for a particular disease. Working closely with patients is crucial in crowdfunding efforts, pharmaceutical drug development programmers, clinical trials, and scientific collaborations. It is also required to improve healthcare service delivery across sectors. In developed economies, the role of patients and patient organizations in combating rare diseases has grown; less so in India.
Orphan Drugs in India: Need to Redesign Access
Less than one in 10 patients worldwide receives precision treatments even for recognized rare conditions that have one or more standardized treatment protocols. This massive treatment gap is a matter of urgent concern. Indeed, rare diseases are neglected to an extent that drugs used for their treatment are called ‘orphan drugs’. Even today, there is lack of proper regulation of orphan drugs in India, which hampers their indigenous development. Though India has a higher rare diseases population than the world average, it still lacks a national legislation for orphan drugs. Nor are such drugs profitable for drug companies since rare diseases affect a relatively small number of people. The new ‘Drug and Clinical Trial Rule’, 2019 is a step forward, as it offers incentives for the manufacture of orphan drugs, and encourages private enterprises and start-ups to invest in developing diagnostics, medicines and other products for rare diseases.
However, many factors complicate the drug development process for these diseases, such as lack of clear understanding of patho-physiology, lack of standard comparator drugs, paucity of scientific evidence, and absence of validated preclinical models. The high cost of drug development is also a significant barrier. Despite notable progress in recent years, there remain many obstacles that hinder access to, and use of, these drugs – such as challenges in assessing clinical relevance and cost-effectiveness, high prices, deficient diagnostic systems, inadequate patent protection and product liability, as well as lack of knowledge and training.
Without incentives or interventions by regulatory bodies, the pharmaceutical industry may not be motivated to develop cures for rare diseases, as there is little return on investment. Again, equitable access to drugs is made more difficult by socioeconomic status within societies, age, gender, and availability of caregivers.
The Case for Global Data Sharing
Electronic health data has been a game changer globally. Maintaining digital records not only empowers patients but also helps advance research, particularly in rare diseases. Genomic and clinical data are vital to understanding and mitigating rare diseases, considering that 70–80 percent of such diseases are genetic. For datasets to reveal crucial information, however, a high volume of genomic data needs to be analyzed, with several of these diseases regionally identified and isolated individually. However, the complexities in data policies and regulatory frameworks across countries make direct data-sharing difficult. A federated data system that allows for both local autonomy and global innovation can offer a feasible solution.
Indeed, the case for a global data registry is not merely ethical but also an economic one. Human and monetary losses from misdiagnosis, loss of quality of life, mortality and morbidity, and lack of scientific treatment plans, have long-term impacts on global economies. Moreover, rare diseases are universally a driving contributor to under-5 mortalities, constituting almost 30-35 percent of all such deaths. Therefore, early screening and detection of rare diseases is vital to mitigating perinatal and neonatal diseases.
Policy Challenges for India
In India, there is lack of awareness about rare diseases not only among the general population but also healthcare providers and policymakers. Compared to developed countries like the US, India is lagging by almost 40 years in recognizing the importance of tackling rare diseases. In developing-country settings, much of the effort in mitigating such diseases is focused on accessing orphan drugs. Little attention has been paid to developing registries, genetic screening, access to therapy, health insurance coverage, and public perception about such diseases.
India’s traditional testing and screening mechanisms for genomic assessments in prenatal diagnosis are limited. The 2021 policy focuses on reducing the birth of affected fetuses by using ultrasound and biochemical screenings during pregnancy, and promoting genetic compatibility testing between couples. This requires extensive training of community-level care workers such as the accredited social health activists (ASHAs) as well as equipping primary and secondary-level health centers with specialists. Given the massive human resources shortage in healthcare in the country, there is no plan so far to do either.
Diagnosis of almost all rare diseases takes place only at tertiary health centers, which are concentrated in urban areas. This leaves many cases undiagnosed and is also a loss of accessible resources for advanced testing tools. India is also undergoing a demographic and epidemiological shift in its population’s health needs. Historically, health policy in the country has focused more on infectious diseases, than those lifestyle-related or genetic. Financing priorities have still not changed, while the dynamics of non-communicable diseases have. The management of rare diseases needs to be made more systematic.
The NPRD was formulated to reduce the incidence and prevalence of rare diseases by tackling management challenges through a comprehensive and holistic preventive strategy, and strengthening the knowledge generated through research. It focuses on three core areas: financial support to lower the cost of treatment of rare diseases, early diagnosis, and indigenous research. However, it falls short of building a solid centre-state collaborative framework. This is evident across several provisions of the policy. For example, the National Registry for Rare Diseases, which was designed to create a database of rare diseases in India through trained specialists across designated centres, has approved institutions in only 12 states and four union territories. Even some of the bigger states are left out; thus the registry has made little progress in data collection. Further, there is no mechanism to inform caregivers of further stages of testing or referrals to specialists.
Healthcare services for rare diseases are largely inaccessible to the majority of patients in India, both due to their high cost and the difficulty of finding the right expertise. The NPRD divides these diseases into three groups: those that are amenable to one-time curative treatment; those that need long-term/lifelong treatment courses that are relatively inexpensive; and those whose treatments are of steep cost. Patients in Groups 2 and 3, who require long-term supportive therapies, have to struggle to get state funds or crowdfunding.
Conclusion
The imperative for India is to have a decentralized approach to rare diseases, utilizing epidemiological data to set policies and legislation. Such policies should enable electronic health data recording and sharing, while providing adequate provisions for privacy. Evidence from advanced economies shows that patients struggling with rare diseases are nearly 90 percent more likely to share their data for further research than others. India should also aim to create, protect and promote such recording practices. Policies should expedite scientific innovation and advanced clinical research, and enable key stakeholders such as patients, doctors and caregivers, and the pharmaceutical industry, to easily interact with one another.
The National Digital Health Mission (NDHM) can help improve diagnostics of rare diseases. Various cutting-edge diagnostics have been developed during the COVID-19 pandemic which can be repurposed for rare diseases. However, Indian experts believe that it will be difficult to scale these capabilities to the local level, even as they are already widely used by the country’s bio-community. Traditional mindsets will have to shift for treatments to change and incorporate the recent technological developments in multiple ways.
Most rare diseases require lifetime treatment and support for the patient to maintain a decent quality of life. Caregiving drains families physically, emotionally and financially. Therefore, there is also need to integrate palliative care, rehabilitation, and counselling services as an integral part of the management of rare diseases.
Substantial resources are required, as is the active support of government. It can be difficult for countries such as India to divert resources from the more prevalent general diseases to rare diseases. Yet, lower prevalence should not mean less significance. Implementing the NPRD is only one step in addressing the challenges in managing rare diseases.
Annexe
Status of Rare Diseases Today: Panel Discussion
28 February 2022
Speakers
Prasanna Shirol, Co-Founder and Executive Director, Organization for Rare Disease India
Sheffali Gulati, Chief, Child Neurology Division, Department of Pediatrics, AIIMS, New Delhi
Vijay Raghavan, Principal Scientific Adviser to the Government of India
Craig Martin, CEO, Global Genes, USA
Craig Lipset, Co-Chair, Decentralised Trials and Research Alliance (DTRA), USA
Shamika Ravi, Vice President, Economic Policy, ORF
The discussion video can be accessed here.
The authors would like to thank Parvathy Krishnan, Oommen C Kurian, Vijay Sappani, and Kristin Kantautas for their inputs on an earlier draft.
About the Authors
Mona is a Junior Fellow with the Health Initiative at ORF.
Shubhangi Patel is a Research Intern with the Health Initiative at ORF.
Link for original articlehttps://www.orfonline.org/research/rare-diseases-in-india-orphan-no-more/?amp :